Byron Purse
Professor, Organic Chemistry
JDP Advisor
office: CSL 213
phone: 619-594-6215
email: bpurse@sdsu.edu

Purse Group Page
Curriculum Vitae
- Professor, San Diego State University, 2022—present.
- Associate Professor, San Diego State University, 2018—2022.
- Assistant Professor, San Diego State University, 2013—2018.
- Assistant Professor, University of Denver, 2008—2013
- Postdoctoral Research Associate, Bäckvall Group, Stockholm University, Sweden, 2006—2008
- PhD in Organic Chemistry, Rebek Group, The Scripps Research Institute, La Jolla, California, 2000—2005
- BSc High Honours in Chemistry and Biochemistry, University of Regina, Canada, 1996—2000.
Research Interests
In the Purse Lab, we are interested in using molecular design and synthetic organic chemistry to create new molecules that can serve in practical applications and be used to study the fundamental relationships between molecular structure and properties. With new molecules in hand, we use the tools of modern physical organic chemistry to study their properties, including self-assembly, molecular encapsulation, fluorescence, and the ability of some of our molecules to functional as molecular probes or prospective medicines. Students working in the Purse Lab can expect to gain hands-on experience in current synthetic methodology, organic compound characterization, methods for studying the kinetics and thermodynamics of reactions and molecular complex formation (NMR, ITC, optical spectroscopy), and recently biochemistry! We are always interested in new co-workers with a passion for knowledge, a drive for success, a creative mindset, and a dedication to the pursuit of excellence.
Unnatural Nucleosides
Nucleosides are the building blocks of DNA and RNA, the molecules that store the genetic code. In addition to this role, they are used as the energy currency of cells (ATP is a nucleoside triphosphate), for controlling chemistry in cells (catalytic RNA in ribosomes), and as chemical transporters (tRNA). Their importance cannot be understated! Chemists have an interest in modifying the structures of nucleosides to make new molecular probes (e.g. fluorescent molecules that can be used to track and study nucleic acids) and to make drugs. Many important medicines are synthetic derivatives of nucleosides that “trick” infected or malignant cells into using the “unnatural” nucleoside by mistake during replication. This mechanism is how azidothymidine (AZT) attacks the HIV virus, gemcitabine kills breast cancer cells, and acyclovir slows the spread and growth of the Herpes virus. Back to the former application, fluorescent nucleoside analogues are used to study how enzymes in our body work and for modern, next-generation DNA sequencing methods.
Our lab is interested in developing new nucleoside analogues both for unmet needs as molecular probes and in medicinal chemistry. We are currently working on synthetic elaboration of natural nucleosides to make the nucleobases fluorescent, while minimizing the biochemical impact of the required structural changes. One of the applications of these compounds is to create a fluorescent version of DNA using the PCR reaction! New compounds designed and prepared by our lab have promising photophysical properties, and we are currently testing their applications as molecular probes. Relatedly, we are examining chemical methods for delivering analogues of nucleoside triphosphates across the plasma membranes of living cells. These methods are designed for delivering both molecular probes and drugs. As such, we are contributing new tools for understanding biology and testing new approaches to treat disease.
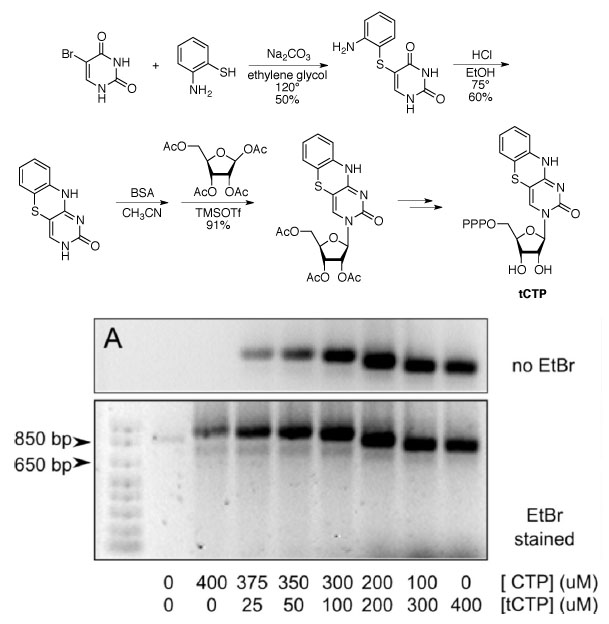
Our lab has prepared a tricyclic derivative of cytidine, dCTP, to make a fluorescent mimic of the natural RNA building block CTP. The synthetic molecule can be used as a substrate for transcription using T7 RNA polymerase to produce fully fluorescent RNA that can be visualized without the toxic stain ethidium bromide, EtBr (see reference 2).
Supramolecular Chemistry
The field of supramolecular chemistry is concerned with the study of how and why molecules can self-assemble into higher-order structures with emergent properties. Throughout the history of the field, most studies and applications have used assemblies at equilibrium, where there is dynamic exchange of components between assemblies. Our group is interested in self-assembled supramolecules with high kinetic stability; that is, those whose components exchange slowly or not at all. Such assemblies can be useful in environments where component exchange cannot be tolerated, such as open systems with flow.
Recently, we have identified the most kinetically stable known molecular capsules that are held together by hydrogen bonds. These capsules will indefinitely trap their occupants at ambient temperatures even as thermodynamics would prefer the release of the capsules' guests. Heat is required to break the capsules apart and release the small guests. Noting the special stability of these capsules, we have turned our attention towards embedding these capsules in polymers so as to harness mechanical forces to control occupancy. The long-term goal of our work in this area is to create new smart materials that can release or take up specific small molecules in response to mechanical forces, for example, to modulate the properties of polymers or to indicate and measure polymer stress that might lead to material failure. We are also investigating the use of our capsules to control chemicals reactions by compartmentalization, similarly to how cells separate chemical environments using their organelles.
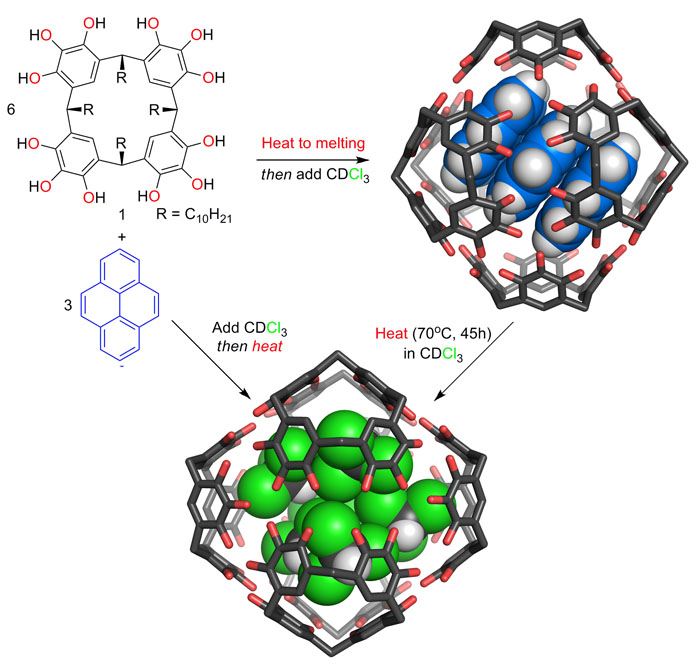
While investigating systems for the entrapment of small molecules in synthetic molecular capsules, we discovered the hydrogen bonded assemblies of pyrogallolarene are the most kinetically stable known capsules of their kind. Unlike for most self-assembling systems, the order of reagent addition and heating controls which assembly is formed and the resulting structure is stable until disassembly is induced by heating (see reference 5).
Selected Publications
- "Sequence-Specific Fluorescence Turn-On Sensing of RNA by DNA Probes Incorporating the Tricyclic Cytidine Analogue DEAtC,"
Turner M.B., Cizmic J.M., Rosansky D.B., Ceja J., Patterson M., Kilcoyne S., Thurber K., Kim G., Dwyer T.J., Purse B.W.,
Bioconjugate Chemistry 34, 1061-1071 (2023). (doi: 10.1021/acs.bioconjchem.3c00134.) - "Live-Cell RNA Imaging with Metabolically Incorporated Fluorescent Nucleosides,"
Wang D., Shalamberidze A., Arguello A.E., Purse B.W., Kleiner R.E.,
Journal of the American Chemical Society 144, 14647-14656 (2022). (doi: 10.1021/jacs.2c04142.) - "Nucleoside analogue inhibitors for Zika virus infection,"
Bernatchez J.A., Coste M., Purse B.W., Siqueira-Neto J.L.,
Zika Virus Impact, Diagnosis, Control, and Models: Volume 2: The Neuroscience of Zika Virus , 385-396 (2021). (doi: 10.1016/B978-0-12-820267-8.00037-6.) - "Single-molecule fluorescence detection of a tricyclic nucleoside analogue,"
Samaan G.N., Wyllie M.K., Cizmic J.M., Needham L.-M., Nobis D., Ngo K., Andersen S., Magennis S.W., Lee S.F., Purse B.W.,
Chemical Science 12, 2623-2628 (2021). (doi: 10.1039/d0sc03903a.) - "Fluorescent Tricyclic Cytidine Analogues as Substrates for Retroviral Reverse Transcriptases,"
M. B. Turner, B. W. Purse,
ChemPlusChem 85, 855-865 (2020). (10.1002/cplu.202000140.) - "Structure-based design of guanosine analogue inhibitors targeting GTP cyclohydrolase IB towards a new class of antibiotics,"
George N.Samaan, Naduni Paranagama, Ayesh Haque, David A. Hecht, Manal A. Swairjo, and Byron W. Purse,
Bioorg. & Medicinal Chem. lett. 30, 126818 (2020). (10.1016/j.bmcl.2019.126818.) - "H-2 NMR reveals liquid state-like dynamics of arene guests inside hexameric pyrogallol[4]arene capsules in the solid state,"
Irazema J. Islas, Dillan Stengel, Cesar A. Garcia, J. Bennett Addison, George N. Samaan, Gregory P. Holland, and Byron W. Purse,
Org. Chem. Front. 6, 1361-1366 (2019). (doi: 10.1039/C9QO00232D.) - "Activity of Selected Nucleoside Analogue ProTides against Zika Virus in Human Neural Stem Cells,"
Jean A. Bernatchez, Michael Coste, Sungjun Beck, Grace A. Wells, Lucas A. Luna, Alex E. Clark, Zhe Zhu, David Hecht, Jeremy N. Rich, Christal D. Sohl, Byron W. Purse, and Jair L. Siqueira-Neto,
Viruses 11, 365 (2019). (doi: 10.3390/v11040365.) - "Electronic modifications of fluorescent cytidine analogues control photophysics and fluorescent responses to base stacking and pairing,"
Teppang, Kristine L.; Lee, Raymond W.; Burns, Dillon D.; Turner, M. Benjamin; Lokensgard, Melissa E.; Coste, Michael; Cooksy, Andrew L.; Purse, Byron W.,
Chem. Eur. J. (2018). (doi: 10.1002/chem.201803653.) - "Fluorescence turn-on sensing of DNA duplex formation by a tricyclic cytidine analogue,"
Burns, Dillon D.; Teppang, Kristine L.; Lee, Raymond W.; Lokensgard, Melissa Eleanor; Purse, Byron W.,
J. Am. Chem. Soc. 139, 1372—1375 (2017). (doi: 10.1021/jacs.6b10410.)
