B. Mikael Bergdahl
Associate Professor, Synthetic Organic, Bio-organic Chemistry
office: GMCS 213G
email: bbergdahl@sdsu.edu

Curriculum Vitae
- B.S/M.S. Chalmers University of Technology, Gothenburg, Sweden, 1987. Advisor: Prof. David Tanner
- Ph.D. Chalmers University of Technology, Gothenburg, Sweden, 1992. Advisor: Prof.'s Martin Nilsson and Thomas Olsson
- Post. doc. University of Notre Dame. Advisor: Prof. Paul Helquist
- Visiting scientist and lecturer. University of California, San Diego and Scripps Research Institute. Advisor: K.C. Nicolaou
- Assistant Professor at San Diego State University 1999-2005
- Associate Professor at San Diego State University since 2005
Research Interests

The main focus of my research is the total syntheses of natural products and development of new chemical methodology in synthetic organic chemistry. My group is also focusing on the synthesis and evaluation of biologically active target compounds having effects against various forms of cancer, rheumatoid arthritis, infectious diseases and hepatitis C. Azaspirene (Project 1), a member of the pseurotin family, represents a novel and unique anti-cancer agent, extraordinary in its ability to inhibit angiogenesis. Rather than destroying cancer cells via conventional chemotherapy and radiation, azaspirene turns off blood supply signals sent out by the tumor cells. My research is to develop the means to chemically make azaspirene and pseurotin analogs with potential properties against cancer or arthritis. My research intertwines development of specifically new methodology in organic synthesis and new strategies toward relevant target molecules, e.g. polyketide natural products against cancer and malaria (Project 2). The total synthesis of a defined target molecule could play an important role in identifying such new candidates in medical treatments.
Specific research interests include the development of new chemical methodologies in asymmetric synthesis employing organometallic reagents, e.g. Zr, Cu, Zn, Si with the emphasis in catalytic reactions using chiral ligands. Novel organocopper reactions have been used to solve key structures such as optically active acetate aldol products found in the pertinent target molecules. Moreover, novel asymmetric remote vinylogous Mukayama aldol reactions have been employed to solve key structures of fragments of polyketide natural products (Project 2).
Project 1. Angiogenesis Inhibitors against Cancer.
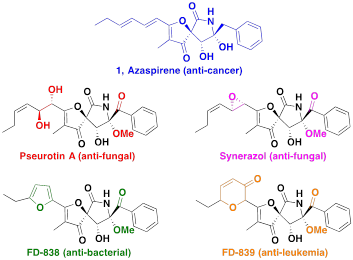
Azaspirene, an effective angiogenesis inhibitor isolated from the soil fungus Neosartorya sp., may be used as a milder form of chemotherapy. Angiogenesis is the body's natural process of forming blood vessels for normal growth and wound repair but can be hijacked by tumors to induce irregular blood vessel growth, which allows the tumors to grow and metastasize. Azaspirene has been shown to block the chemical signaling pathways used to grow irregular tumor blood vessels without disrupting normal blood vessel growth in the body. Azaspirene belongs to the Pseurotin family of compounds whose members share a similar spirocycle backbone and show promise as anti-fungal and anti-bacterial agents. The supply of azaspirene that has been harvested from natural sources is very small and unable to sustain further research; therefore, it is critical that an economical and efficient synthesis be established.
Project 2. Macrocyclic polyketide natural products against cancer and malaria.
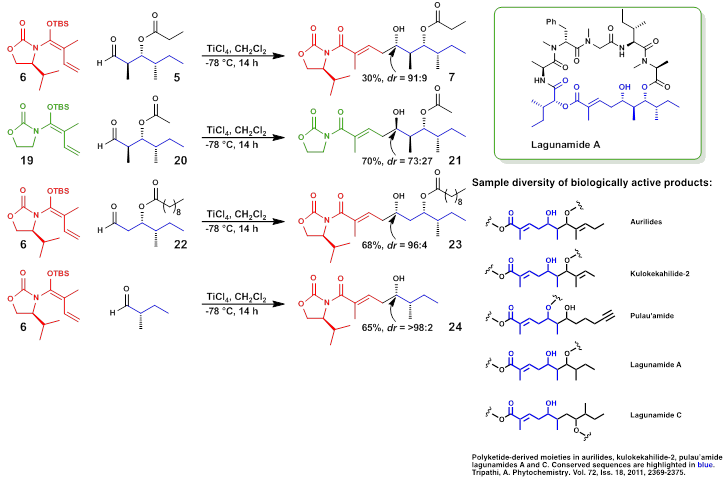
Lagunamides are complex novel macro-cyclic depsipeptides obtained from the marine cyanobacterium Lyngbya majuscula. These molecules possess high anti-malarial properties (IC50 0.19-0.91 mM), cytotoxic properties against leukemia cell lines (IC50 6.4-20.5nM), and colon cancer (1.6nM), respectively. The focus of this research is to develop an efficient and highly convergent strategy for the total synthesis of lagunamides. Vinylogous Mukaiyama Aldol Reactions (VMARs) were used to establish four contiguous stereocenters of the southern hemisphere of the molecule. An efficient synthetic pathway toward Lagunamides will ultimately lead to structural analogs that can be used in quantitative structure-activity relationship (SAR) studies against various cancer cell lines and the treatment of malaria.
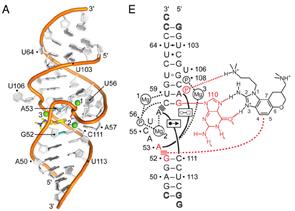
Project 3. Crystal Structure Based Library of New Small Molecules Active Against the Hepatitis C Virus (HCV).
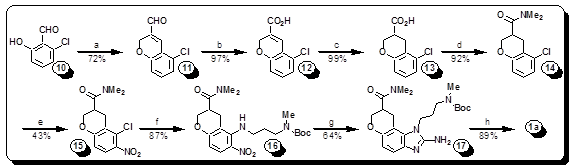
Hepatitis C virus (HCV) has infected some 200 million people worldwide and is major threat to human health. The Interferon-alpha/ribavirin treatments of HCV are unfortunately associated with serious side effects and are effective only in few cases. Recently a class of small drugs was discovered that acts against HCV in a new way, binding to a part of the highly conserved 5' untranslated region of the viral RNA. We reported a novel synthesis and crystal structure of one of these molecules bound to the viral RNA. Our synthetic route offers higher unprecedented access to new and highly optimized drugs against HCV. By utilizing information gained from the crystal structure, we are able to direct our synthesis towards molecules with a higher probability of increasing potency of our novel compounds. Our library currently has two directions for increasing affinity; to make conformationally restricted analogs, and to change ligands that will optimize amine-phosphate salt bridges seen in the crystal structure. New compounds will be tested against the IRES-Iia subdomain by our specially designed fluorescence resonance energy transfer (FRET) assay designed by our collaborators at UCSD, and tested against HCV-infected cells for anti-viral activity and cytotoxicity at various concentrations by collaborators at SDSU allowing us to iteratively refine SAR.
Working in the Bergdahl group.
Students working in my laboratory are exposed to a variety of scientific techniques: Advanced synthetic organic chemistry such as the invention of new chemistry, synthesis of chiral ligands and complex natural products, NMR spectroscopy to determine absolute stereochemistry of optically active compounds as well as determining reaction mechanisms and elusive reaction intermediates. In order to conduct chemical reactions under air-free conditions, my students are trained to handle compounds in inert- atmospheres using manifolds and Schlenk apparatus. The students in my group are also trained to characterize new compounds using spectroscopy (NMR, IR, MS) and flash- chromatography in order to make multi-gram quantities needed, particularly for long synthetic sequences. These are furthermore fundamental skills expected by a majority of pharmaceutical companies, particularly if the goal is to produce high yields as well high purity of products. My research group also has extensive international as well as domestic research collaboration, e.g. University of Melbourne Australia, Scripps Research Institute in La Jolla as well as many other Universities in California encompassing chemistry and biology.
Graduate students graduated from my group, either with a M.S. or Ph.D. degree, benefit from a comprehensive preparation provided by me as the mentor and the knowledge from a critical mass of graduate students from various areas of organic chemistry. My students are also trained to take their own initiatives in the laboratory setting, which has sometimes resulted in publications from this view of serendipity. My group also publishes regularly and we thrive to have those articles submitted to high- profile and internationally respected journals. Graduate students have accepted position in the pharmaceutical industry such as Eli Lilly and Pfizer. Undergraduates from my group have accepted jobs using their synthetic organic skills, or to graduate programs at institutions such as USC, the Scripps Research Institute, and UC San Diego.
Past and present group members
Recent Publications
- "The Conformations of Virginiamycin M1 Diacetate, an Inhibitor of Guinea Pig Brain CCK-B Receptors, in Selected Solvents,"
Kevin Walsworth, Anastasiya Bender, Frances Separovic, Mikael Bergdahl and Robert P. Metzger,
Aust. J. Chem. 73, 230-35 (2020). (doi: 10.1071/CH19577.) - "Syntheses and Binding Testing of N1-Alkylamino-Substituted 2-Amino-benzimidazole Analogues Targeting the Hepatitis C Virus Internal Ribosome Entry Site,"
David Schmit, Urszula Milewicz, Mark A. Boerneke, Scott Burley, Kevin Walsworth, Joann Um, David Hecht, Thomas Hermann, and B. Mikael Bergdahl,
Aust. J. Chem. 73, 212-21 (2020). (doi: 10.1071/CH19526.) - "Water Mediated Wittig Reactions of Aldehydes in the Teaching Laboratory: Using Sodium Bicarbonate for the in Situ Formation of Stabilized Ylides,"
Michael J. B. Kelly, Lucas B. Fallot, Jeffrey L. Gustafson, and B. Mikael Bergdahl,
Journal of Chemical Education 93, 1631-1636 (2016). (doi:10.1021/acs.jchemed.6b00206.) - "Further insight into the asymmetric vinylogous Mukaiyama aldol reaction (VMAR); application to the synthesis of the C27-C45 segment of lagunamide A,"
Brent A. Banasik, Lee Wang, Arielle Kanner, and B. Mikael Bergdahl,
Tetrahedron 72, 2481-2490 (2016). (doi:10.1016/j.tet.2016.03.079.)
MS Theses and Ph.D. Dissertations (2010-2020)
- Lee Wang, Ph.D. Thesis 2019. Total Synthesis of Marine Natural Products: Micromide, Its Analogs, and Lagunamide A.
- Michael Kelly, Ph.D. Thesis 2019. Steps Away from (–)-Azaspirene: Synthesis of the Core Spirocycle Chemical Studies in Copper(I) Iodide Dimethyl Sulfide Catalyzed Asymmetric Conjugate Addition. Wittig Chemistry in the Teaching Laboratory: A Novel Water-Organic Interface Reaction.
- Paul Smith, Master's Thesis 2016. Progress Towards an Improved Total Synthesis of Azaspirene, a Powerful Angiogenesis Inhibitor and Copper Catalyzed Conjugate Silyl Additions to Enones.
- Arielle Kanner, Master's Thesis 2016. Chemical Studies of the Remote Asymmetric Vinylogous Mukaiyama Aldol Reaction and its Application Towards the Total Synthesis of Lagunamide A.
- Brent Banasik, Ph.D. Thesis 2016. Total Synthesis of Lagunamide A via Remote Vinylogous Mukaiyama Aldol Reactions, Chemical Studies Toward the Total Synthesis of Micromide and Preliminary Studies Toward the Total Synthesis of Azaspirene.
- Tim Montgomery, Ph.D. Thesis 2016. Part I: Total Synthesis of (-)-Azaspriene Part II: Alkenylzincate and Disilylzinc Conjugate Additions Catalyzed by Copper(I) Iodide Dimethyl Sulfide Complex.
- Lucas Fallot, Master's Thesis 2014. “On Water” Wittig Reaction Laboratory Experiment and the Development of an “On Water” Catalytic Wittig Reaction.
- David Schmitt, Ph.D. Thesis 2014. Progress Toward a Scalable Synthesis of Azaspirene, An Angiogenesis Inhibitor & Synthesis of 2-Amino-benzimidazole Compounds Targeting Subdomain IIa of the Internal Ribosome Entry Site Inhibiting Translation of The Hepatitis C Virus.
- Urszula Milewicz Master's Thesis 2014. Novel Approach to Synthesis and Analog Diversification of New Hepatitis C Inhibitors.
- Scott Burley, Master's Thesis 2013. Diversity - Optimized Route to the Ergoline Skeleton and the Efficient Synthesis of New HCV Inhibitors.
- Isabelle Nevchas, Master's Thesis 2010. Chemical Methodology toward the Total Syntheses of Virginiamycin M1 and Micromide Natural Products.
