Venkatesan S. Thimmakondu
Adjunct Professor, Physical Chemistry
office: CSL-312B
email: vthimmakondusamy@sdsu.edu

Curriculum Vitae
- Adjunct Faculty, San Diego State University, USA, 2018 to present.
- Principal Investigator (affiliate), SETI Institute, USA, 2017 to present.
- Assistant Professor, BITS-Pilani, Goa Campus, India, 2013-2016.
- Post-Doctoral Fellow, The University of Texas at Austin, USA, 2010-2013.
- Post-Doctoral Fellow (AvH fellowship), Technical University of Munich, Germany, 2008-2010.
- Ph.D., University of Hyderabad, India, 2002-2007.
- M.Sc., Madurai Kamaraj University, India, 1999-2001.
- B.Sc., Madurai Kamaraj University, India, 1996-2001
Research Interests
1. Molecules with a planar tetracoordinate carbon (ptC) or silicon (ptSi) atom
Tetrahedral tetracoordination is one of the fundamental paradigms of organic chemistry. But, there are also molecules with a ptC/ptSi atom, where this rule is disobeyed.1-3 The fundamental fact is, no two structural isomers exhibit similar chemical properties. Hence, preparing these molecules is an essential first step in understanding what kind of physical and chemical characteristics it might possess. Although our efforts are largely theoretical at the moment in this topic, we are in touch with different experimentalists (from gas-phase molecular spectroscopists to synthetic organic chemists) on a continuous basis to realize how one can make these compounds.
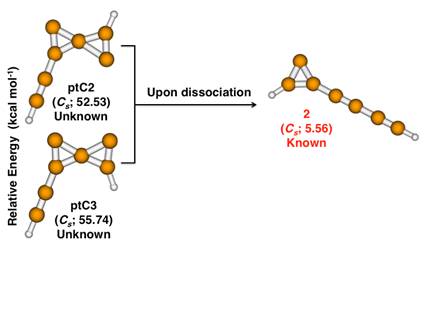
In C7H2 elemental composition, we have theoretically proved that molecules with a ptC atom serve as reactive intermediates to carbenes1. In this work, we use high-level ab initio methods to establish the link between the ptC molecules (ptC2 and ptC3; on the left) and an experimentally known carbene molecule [1-(buta-1,3-diynyl)cyclopropenylidene (2) ; on the right]. The relative energies are calculated using coupled-cluster approximation and the differences are taken from heptatriynylidene (1) (the linear triplet isomer of C7H2) molecule. In Si2C5H2, the putative global minimum itself contains a ptC atom3. However, the latter isomer remains elusive in the laboratory to date.
Key Publications:
[1] K. Thirumoorthy, A. Karton*, and V. S. Thimmakondu*, From high-energy C7H2 isomers with a planar tetracoordinate carbon atom to an experimentally known carbene, J. Phys. Chem. A, 2018, 122, 9054–9064. DOI: 10.1021/acs.jpca.8b08809
[2] V. S. Thimmakondu* and K. Thirumoorthy, Si3C2H2 isomers with a planar tetracoordinate carbon or silicon atom(s), Comput. Theoret. Chem. 2019, 1157, 40–46. DOI: 10.1016/j.comptc.2019.04.009
[3] K. Thirumoorthy, A. L. Cooksy, V. S. Thimmakondu*, Si2C5H2 isomers – search algorithms versus chemical intuition, Phys. Chem. Chem. Phys. 2020, 22, 5865–5872. DOI: 10.1039/C9CP06145B
2. Interstellar chemistry
Identifying molecules many light years away is an open challenge to the scientific community. So far, approximately 209 molecules have been identified in space to date. Some of them are normal molecules such as, water, ammonia, and carbon monoxide but there are also other strange molecules such as, cyclopropenylidene and propadienylidene (isomers of C3H2). Understanding their existence far away in space is one of the first steps towards understanding the origin of life itself.
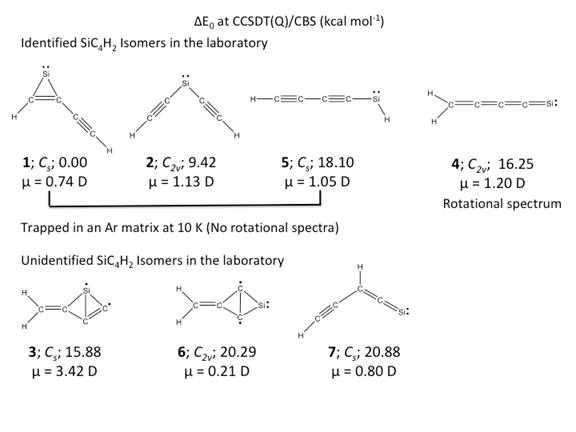
The challenges of identifying molecules in non-terrestrial environments could only be completely resolved by the synthesis and characterization of these new (non-terrestrial) molecules in terrestrial environments (on planet Earth). Although radioastronomers observe rotational transitions in space, we need a match to confirm the presence of the exact same molecule on earth. Needless to say, quite exotic molecules have been detected in the interstellar medium (ISM) along with simple molecules. The challenges associated with the synthesis and identification of these new molecules on earth is one of the primary reasons why only 209 molecules have been confirmed so far in space, instead of a greater number. We are currently working on various elemental compositions [for example, CnH2 (here n = 5, 7, and 9) and SiC4H2] for which some experimental data are already available. However, we note that all the hypothetical isomers proposed by us even on the low-energy region on these elemental compositions remain elusive in the laboratory to date. We believe that our current theoretical studies may eventually facilitate laboratory detection of these previously unobserved isomers.
Key Publications:
[1] N. Job, A. Karton, K. Thirumoorthy, A. L. Cooksy, and V. S. Thimmakondu*, Theoretical studies of SiC4H2 isomers delineate three low-lying silylidenes are missing in the laboratory, J. Phys. Chem. A, 2020, 124, 987–1002. DOI: 10.1021/acs.jpca.9b11742
[2] K. Thirumoorthy, M. Viji, A. P. Pandey, T. Netke, B. Sekar, G. Yadav, S. Deshpande, V. S. Thimmakondu*, Many unknowns below or close to the experimentally known cumulene carbene ~V A case study of C9H2 isomers, Chem. Phys. 2019, 527, 110496. DOI: 10.1016/j.chemphys.2019.110496
[3] V. S. Thimmakondu*, MgC2H2 isomers — Simple penta-atomic molecules missing in the laboratory, Chem. Phys. 2020, 538, 110899. DOI: 10.1016/j.chemphys.2020.110899
(3) Flat Crown Ethers
Crown ethers characteristically bind different metal cations in complex solutions depending upon the size of the macrocyclic ring, polarity of the solution, and also the type of the donor atom. Thus, they have been widely used in chemical separations, analytical methods and also in nuclear waste management. In this work, utilizing molecules with ptC or ptSi atoms, new crown ether molecules will be designed. Chelation of these molecules with different metal ions or small guest molecules will be characterized. Preorganizing the host molecule is a necessary step to suit appropriate guest ions or molecules. It is believed that using the advent of molecules with a ptC atom, new crown ether molecules could be characterized and at the same time preorganization would be enhanced. This work is currently ongoing. We have put some of our latest results in the preprint server, ChemRxiv.
V. S. Thimmakondu*, K. Thirumoorthy (2020): Flat Crown Ethers with Planar Tetracoordinate Carbon Atoms. ChemRxiv. Preprint. DOI: 10.26434/chemrxiv.12152184.v1
(4) Organomagnesium Crown Ethers
This is a spin-off idea we got while working on flat crown ethers. Currently we are designing new organomagnesium crown ether molecules considering the synthetic challenges of flat crown ether molecules containing ptC atoms. More information will be added about these molecules as time proceeds.
